Anode and Cathode in Electrolysis
The products at the. Find out everything you want to know about electrolysis the only method recognized as.

Look4chemistry Electrolytic Cell Electrochemistry Cell Redox Reactions
The ion which is discharged at the anode during the electrolysis of copper sulphate solutions using copper electrodes as anode and cathode.

. Answer 1 of 3. The above reports of the divergent paired electrolysis mainly. The electrolysis of acidulated water is considered to be an example of catalysis.
For example in the electrolysis of water acidulated the half -reaction occurring at the anode is 2. However limited attention has been paid to the study of cathode. Ad World-class Electrolysers And Electrode Coatings That Produce Chlor-alkali Products.
Zhang et al. Anode mesh for seawater electrolysis We offers Platinized Titanium Anodes and MMO Anodes for Electrochemical and Metal Finishing Industries. Bichlor Electrolysers Out-perform The Closest Alternative Technology.
The cathode is the current that leaves the electrodes or the cathode is a result of a reduction reaction taking place in an. Anode and Cathode. Trick to find products of electrolysis at cathode and anode Electrochemistry Class 12Test your self solution linkhttpsyoutuben8DktAvRGo8.
A common mnemonic is anode current into the device. Electrolysis is safe permanent effective hair removal for all skin colors. Pb 2 ions gain electrons at the cathode and become Pb atoms.
Cathode is the electrodemetal plate that is connected to the negative terminal of the cell. Westfield Electrolysis The only option for permanent hair removal. Another mnemonic is to note the cathode has a c as does reduction.
A useful mnemonic to remember this is AnOx RedCat Oxidation at the Anode Reduction at the Cathode. The cathode is the electrode where electricity is given out or flows out of. The so-called anode effects and polarization and to avoid the difficulties related to the carbon anode 3-5.
What is anode and cathode in electrolysis. The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. Anode is electron deficient and hence the negative ions are attracted to the anode where they lose electron and become atoms.
The products at the. The electrolysis modality was the first method used to remove hair permanently back in 1875. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment but also produces value-added materials on both sides.
Cathode leaves the electrode current and anode enters the current. Hence reduction at the cathode. The terms were coined in 1834 by William Whewell who derived the words from the Greek word Kathodes which means descent or way down.
B During the electrolysis of copper II sulphate solution using platinum as cathode and carbon as anode i State what you observe at the cathode and at the anode ii State the change noticed in the electrolyte. Used the H-type two-electrode catalytic system to simultaneously produce furoic acid and furfuryl alcohol with the Ni 2 PCFC anode and the Cu 3 PCFC cathode the use of higher anode and cathode potentials caused the side reactions of water splitting in the electrolyte. The term electrolysis branded the process of permanent hair removal.
In contrasts with a cathode an electrode by which conventional current leaves an electrical device. In chemistry a cathode is the electrode of an electrochemical cell at which reduction occurs. An aqueous solution of Na_2SO_4 is electrolysed using Pt electrodes.
To make efficient use of electrical energy in the whole electrocatalysis conversion process the integrating of anode and cathode reactions plays a vital role. Now about anode if cathode is negatively charged so it is easy to remember that anode is positively c. The direction of current in a circuit is opposite to the direction of electron flow.
An anode is an electrode by which the conventional current enters into a polarized electrical device. The cathode is negatively charged to remember how the cathode is negatively charged consider caThode T as a negative so it is easy to remember carhode as a negatively charged. Yun et al6 examined the effect of the configuration of the slits formed on the cathode on the escape of the hydrogen bubbles toward the backside of.
It is the electrodemetal plate that is connected to the positive terminal of the cell. Cu 2 OH SO 4 2- - H. The electrode at which oxidation occurs is called an anode and the one at which reduction occurs is called the cathode whether it is an electrolytic cell or a galvanic cell.
Br ions lose electrons at the anode and become Br atoms which pair up to form Br 2 molecules. Answer 1 of 5. An aqueous solution of Na_2SO_4 is electrolysed using Pt electrodes.
Solid round titanium bars coated with MMO are manufactured based on the life expectancy of the anodesCommon. The anode is the negative or reducing electrode from where electrons are released to the external circuit and oxidize during an. The anode is the electrode where electricity moves into.
When dilute sodium chloride is electrolysed using graphite electrodes the cation is discharged at the cathode most readily. We supply MMO Tube anodes with KYNARHMWPEXLPEPVC cable attached as per customer requirements. The product of electrolysis of concentrated aqueous sodium chloride are sodium hydroxide hydrogen gas and chlorine gas.
Ruth Coutant-Villa LE CPE. Main Office - Private Suite - 908-654-4322. Anode and cathode are defined by the flow of current.

Electrochemistry Featuring Electrolysis And Fuel Cells Chemistry Classroom Electrochemistry Chemistry Lessons

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions

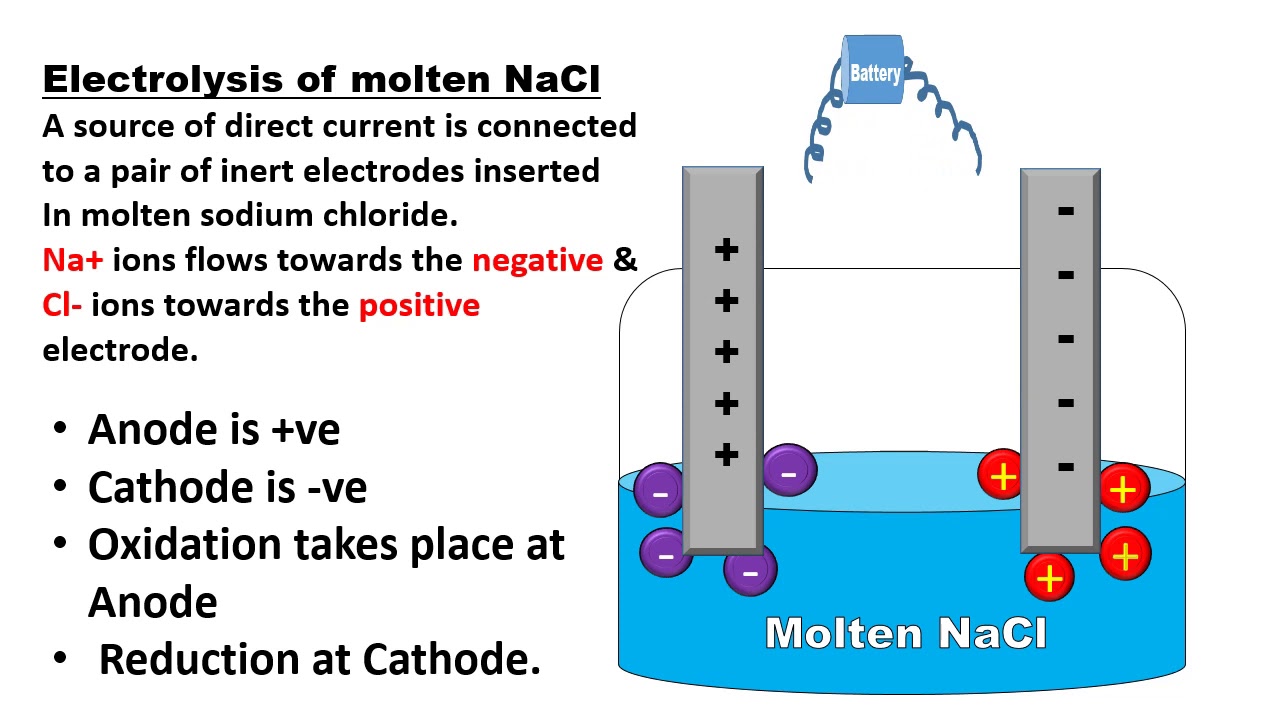
Electrolysis Of Molten Nacl Oxidation Half And Reduction Half Cell Reactions In Electrolytic Cell Quimica
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Piscinas Escuela De Natacion

Electrolytic Cell Electrolysis Of Nacl Notas De Quimica Quimica Matematicas
Comments
Post a Comment